



Sterilisation Consultancy & Process Equipment
Sterilisation Consultancy & Process Equipment
Sterilisation Consultancy & Process Equipment
Sterilisation Consultancy & Process Equipment
Prism Industrial Controls
“Delivering safer, smarter, compliant and streamlined sterilisation solutions to Medical Devices Industry.”
We established Prism Industrial Controls in June 2000 and together with past experience have been involved in Industrial Sterilization for over 30 years.
Our key service is Consultancy related to Ethylene Oxide Sterilization for the Medical Devices Industry.
Our services and products comply with regulated industry requirements in accordance to American and European standards: ISO–11135 ISO / EN–14937 CE / ATEX directives TA Luft emissions standards SEVESO Food and Drugs Administration – FDA 21 CFR part 820 and part 11
Since PrismIC’s foundation we have formed many strong formal relationships with trusted third party suppliers that also allow us to directly offer to our clients numerous process solutions.
We are very proud of our client list and endeavour to always maintain a strong working relationship through excellent service, professionalism and discretion with all our customers.
Why Preconditioning and Degassing?
Investment v's throughput
Three stage process vs single stage process.
Using preconditioning for load preparation prior to charging the sterilisation chamber with product then followed by additional degassing in a separate cell or room after sterilisation is a common method of getting best time utilization of the sterilisation process.
All products are different, requiring different sterilisation approaches.
If we take a typical medical device being processed through the sterilisation chamber only the cycle time could be typically 20 to 24 hours long whereas with the addition of Preconditioning and Degassing outside of the sterilisation chamber the sterilisation cycle time could be reduced to 8 to 10 hours. This means that you can achieve around twice as many cycles per day through the sterilisation process
From a capital investment point of view
A typical preconditioning cell and degassing cell would be around 10 to 15% of the sterilisation chamber value which means that the addition of cells allows for greater utilisation of the most expensive process element the sterilisation chamber.
Challenges to traditional Degassing methods today
1.Removal of EO to ensure the product load is below an explosive level (below LEL).
2. Removal of EO to ensure the product load does not release EO in working areas bringing levels above permissible personnel working levels.
3. Prior to use the actual product contains EO levels lower than permissible levels allowed for patient contact.

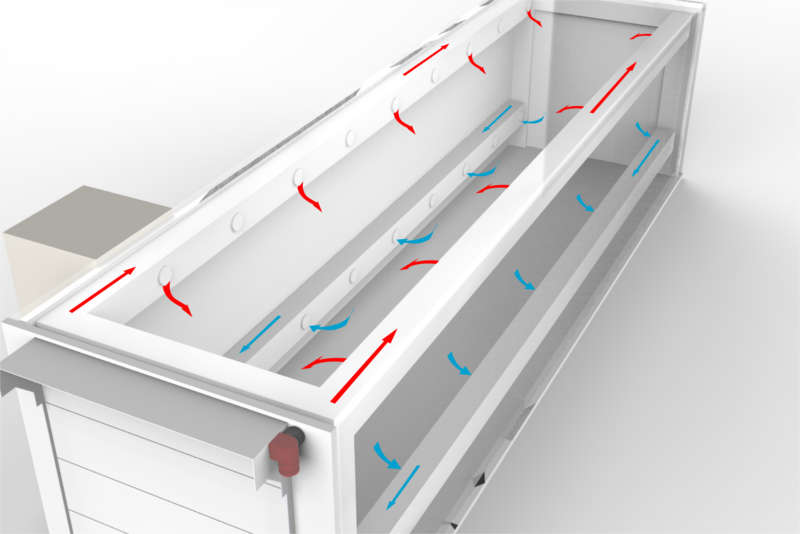
If we consider additional Degassing in a 3-stage Process it is important that Levels 1 & 2 are met* prior to removal from the Sterilisation Chamber and further degassing is performed in a cell and/or room to attain Level 3 levels.
Item 1: the Lower Explosion Limit (LEL) level is a physical EO level that remains the same but both the personnel working levels and patient contact levels are under scrutiny and are destined to be reduced. These changes are a continuous challenge to traditional atmospheric degassing cell and rooms.
These changes are a continuous challenge to traditional atmospheric degassing cell and rooms
* in some processes, an automated transfer from steriliser to the degassing cell with no personnel interaction together with good extract and ventilation allows for products to move into the degassing cell without meeting item 2.
Please email us at info@prismic.ie, or please use contact form.
- Vacuum Degassing
- Preconditioning Cells
- Contact form
